A middle-aged patient without any noticeable comorbidity has been admitted to our ICU due to respiratory failure caused by COVID-19 and had to be intubated.
CT of the thorax was performed five days before our ultrasound examination:
The thorax ultrasound (TUS) exam was performed. For a better description of the probe position use this picture:

The probe was held with the marker pointing towards the head by performing sagittal or coronal sections.
We started the exam ventrally in supine position:

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the region of the left red point
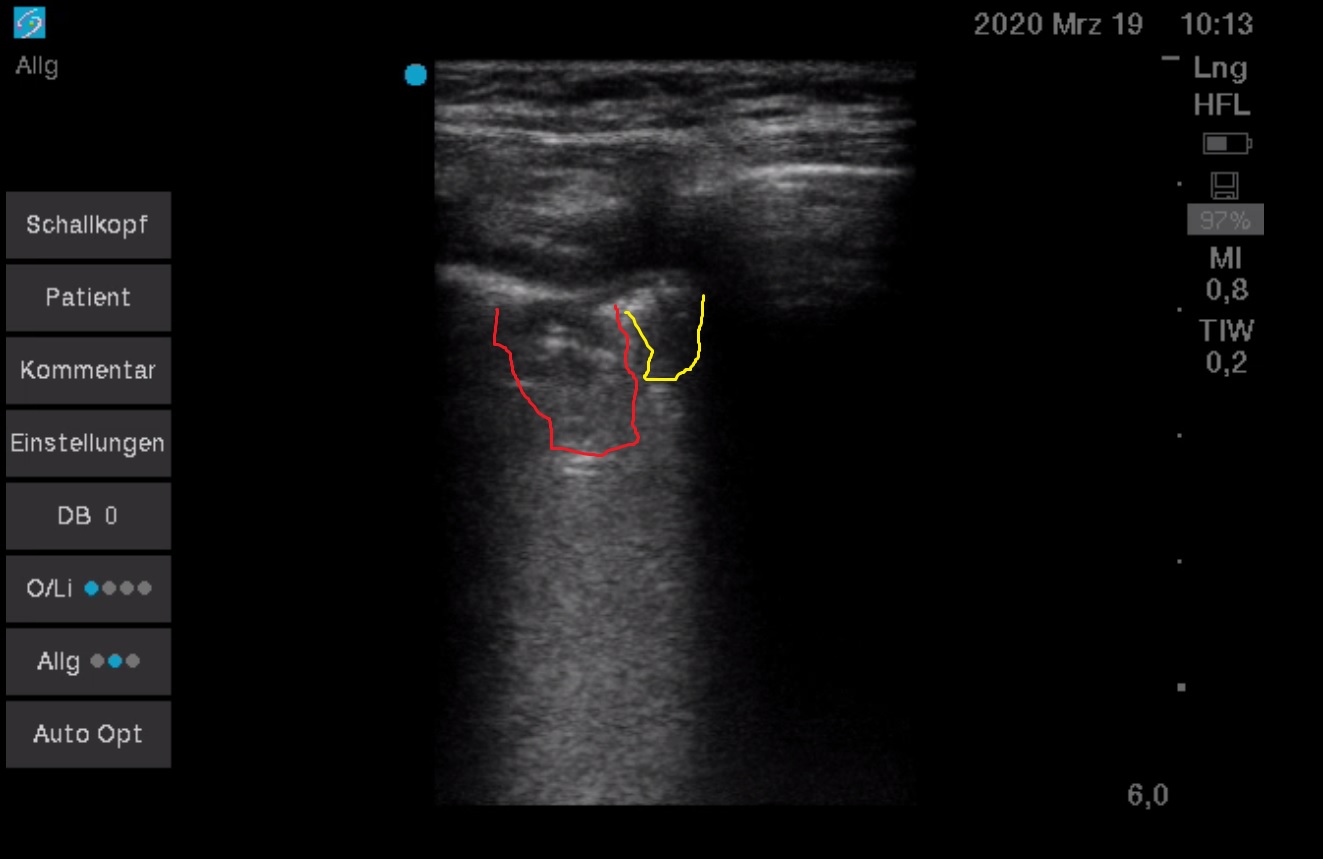
Bat sign displayed. A-Profile, sporadic I-line (yellow). Physiological finding.

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed medially to the left yellow point
Bat sign displayed. A-Profile only in the left part of the Merlin space (blue) = in the space between the ribs shadows (brown) deep to the pleural line (red). An accumulation of B-lines (B3-Profile) in the rest of the Merlin space.
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the left yellow point
Bat sign displayed. About 1 cm-wide subpleural consolidation with a shred line delineating it from the adjacent still aerated lung. The adjacent lung with a high-degree interstitial syndrome – white lung (confluent B-lines displayed as hyperechoic space below the consolidation). In the right part of the Merlin space, there are many B-lines next to each other (B3-Profile).

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed lateral to the left yellow point
A-Profile in the left (cranial) part of the Merlin space (physiological finding). In the middle, there is a subpleural consolidation with a shred line delineating it to the adjacent aerated lung (red). The adjacent lung of white lung character (merging B-lines making this part of Merlin space hyperechoic). In the right part of the image (caudally), there is a trace of a next subpleural consolidation (yellow).
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed laterally and caudally to the left yellow point. Then, the probe is moved caudally, and three adjacent intercostal spaces are displayed. Different findings in Merlin space in each intercostal space (IC) are found:
A-Profile in cranial IC space.
For the finding in middle IC space, see the previous video and picture.
In the caudal IC space, the B3-Profile is alternating with A-profile depending on the probe angulation. While seeing the B-Profile, the pleural line is uneven and thickened.
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the left green point.
Accumulation and even confluence of B-lines giving the picture of a high-degree interstitial syndrome (B3 to white lung). The pleural line seems to be uneven and thickened.



Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the right red point.
A-profile. Occasionally, a short thickening of the pleural line appears (yellow). Sometimes, we may see 1-2 B-lines simultaneously (red). The finding of the next vertical artefacts can not be described as B-lines. The conclusion finding in this IC space is A-profile; however, we have to explore the adjacent lung surface carefully. A-line (yellow).

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the right yellow point.
O-Profile in the left part of the Merlin space with not cleat delineated sub-A-lines and few I-lines (orange). In the right part of the Merlin space, there are confluent or even merging B-lines – high-degree (B3) interstitial syndrome or even white lung (yellow). The pleural line seems to be uneven and thickened.
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the right green point.
In the first displayed IC space, there are confluent to merging B-lines in the Merlin space (B3 interstitial syndrome or even white lung). In the right part of the image (caudally), there is A-Profile with some I-lines.
The probe is then moved cranially, and the adjacent cranial IS space is displayed: here the picture of a high-degree interstitial syndrome (B3 to white lung) and a subpleural consolidation by shred line delineated to the still aerated lung.
The patient was turned into the prone position, and the dorsal surface of his thorax was examined:

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the right blue point
About 2×2 cm subpleural consolidation by shred line (yellow) delineated to the adjacent still aerated lung tissue (green). In the consolidation, there is static punctiform air-bronchogram (red). The adjacent lung below the consolidation as well as subpleural with a high-degree interstitial syndrome (merging B- and sub-B-lines).

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed caudally to the right blue point
The liver and diaphragm, above a short part of the lung surface with A-profile, one I-line (orange) and one B-line (red). Cranially (left) high-degree interstitial syndrome with an uneven and thickened pleural line. Without detection of pleural effusion (but the patient is now lying on the abdomen, so now this is not the most dependent part of the pleural cavity).

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed caudally to the right blue point, the probe was moved medially
The diaphragm and liver, pararenal fat below the diaphragm when moved medially. A short part of the lung surface with A-profile above the diaphragm, more cranially then a high-degree interstitial syndrome with uneven and thickened pleural line and a small subpleural consolidation (yellow).

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the right pink point
A-profile with only sporadic B-lines. In the cranial IC space, in its left (cranial) part, there are merging B-lines (white lung, orange). Minimal angulation and the lateral movement of the probe changes the quality of the picture substantially.
The probe was moved caudally:

Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed caudally to the right pink point and moved further down
A-profile with only sporadic B-lines. Minimal angulation and the lateral movement of the probe changes the quality of the picture substantially. In the caudal IC space, there is a high-degree interstitial syndrome (white lung, green) arising from a short part of the pleural line affected with a small subpleural consolidation (yellow), caudally one B-line (red) and one I-line (orange).
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed in the left blue point
The second IC space above the diaphragm with the high-degree interstitial syndrome in its cranial part. There is also an accumulation of very small subpleural consolidations. Caudally as well as in the first IC space above the diaphragm, there is A-profile with I-lines (as a physiological finding here). Without the detection of pleural effusion.
Intercostal scanning plane perpendicular to the rib and intercostal space axis, probe placed cranially to the left blue point
A subpleural consolidation with sporadic static punctiform air-bronchograms occupies the most of the Merlin space. The interface to still aerated lung represents shred line with high-degree interstitial syndrome below it. The craniocaudal diameter of the consolidation is about 2cm.
The maximal pathological finding can be seen between the blue and green point on the right side of the thorax:
The pleural line is visible as a hyperechoic line in the cranial IC space (displayed at the beginning of the recording), in caudal IC space only sporadic. Most of the IC space depicts subpleural consolidation with static punctiform air-bronchogram and irregular border to the still aerated lung centrally (shred line). The adjacent lung has a high-degree interstitial syndrome. The craniocaudal diameter of the consolidation is about 3-4cm.
The probe was rotated in an anticlockwise direction. The IC space is now displayed in its long axis:
The biggest part of the consolidation is depicted. Irregular border to the adjacent still aerated lung (shred line), static punctiform air-bronchogram. The transversal diameter of the consolidation is about 3-4cm.
While a higher PEEP (of 20 cm H20) for a limited time was applied, the consolidation was observed:
We may see a weakened lung sliding without a detection of a change in neither size nor character of the consolidation. Non-recruitability of such consolidations was demonstrated.
Laterally to this point, we see this picture:
In cranial IC space, the Merlin space is filled by high-degree interstitial syndrome with a subpleural consolidation in its cranial part. One IC space caudally (directly above the diaphragm), there is A-profile.
Conclusion:
- Focal interstitial syndrome scattered on the lung surface at many places (see the formerly described localisations) alternating with A-profile (physiological finding) – so-called spared areas.
- Some subpleural consolidations are imposing as inflammatory infiltrations, the biggest one of 3-4 x 3-4 cm size is located dorsobasally on the right side of the thorax.
- No pleural effusion.
- Lung sliding preserved and pretty good also expressed in cranial parts of the lung.
- One of the consolidations not recruitable. We predict a good clinical effect of prone positioning because of the presence of high-degree interstitial syndrome and its predilection dorsobasally.
Commentary:
The lung injury by COVID-19 pneumonia has been observed to occur under the picture of interstitial pneumonia. In severe cases, the findings may progress in larger consolidation.
Typical findings are:
- Focal interstitial syndrome (IS) = a presence of 3 or more B-lines in one IC space (arising from about 2cm part of the pleural surface, which is depicted by scanning the IC space perpendicular to its long axis). The detection of a true B-line and distinguishing it from other vertical artefacts (e.g. I-, Z- or E-lines) is fundamental for further correct interpretation!
- The interstitial syndrome may be distinguished in:
- B7 (septal rockets) – there are 3-4 B-lines in one IC space 6-7mm apart from each other.
- B3 (ground glass rockets) – there are more than B-lines in one IC space only a few mm (3mm or less) apart from each other, B3 may be a progression of B7-IS.
- Further progression of IS and merging of B-lines results into the picture of so-called highest-degree IS (white lung, Birroleau variant) in which the Merlin is homogenous hyperechoic.
- Distribution of the focal IS on the whole lung surface is not entirely gravitational dependent. Typical are so-called spared areas – parts of lung surface displaying physiological pattern (A-profile) abut the parts of lung surface closely with pathological patterns (IS, consolidations). The distribution of pathological findings in predominantly in dorsal lung lobes, but is not exclusively limited to these parts of the lung, is disperse and bilateral.
- Small subpleural consolidations (1-2 cm) are typical findings by interstitial pneumonia, in severe cases and by superinfections may be larger.
- Static punctiform air-bronchograms may be seen in these small subpleural consolidations. In case of progression (typically because of superinfection), these consolidations become more substantial, and findings typical for typical pneumonia may be seen – static arborescent air-bronchogram, dynamic air-bronchogram or fluid-bronchogram.
- Pleural effusion is not typical for IS pneumonia, and its presence may signalize a complication (e.g. superinfection, heart failure).
- An uneven and thickened (more than 2mm) pleural line, as well as poor perfusion of these consolidations evaluated using doppler, has been described.
Because of a better sensitivity of CT scan the thorax than that of PCR testing from the nasopharyngeal swab and already reported correlations between CT and LUS findings in other viral pneumonia, the LUS may be an option in screening, and early detection of COVID-19 related pneumonia is still asymptomatic patients.
The main CT findings are ground-glass opacities (GGO), crazy-paving pattern and consolidations, all located predominantly in the lower lung lobes in the following stages:
- Early-stage 0.-4.day of the initial symptoms with GGO,
- Progressive stage 5.-8.day with the progress of GGO distribution, crazy-paving and consolidations,
- Maximal stage 9.-13.day with a maximum of pathological changes and
- Absorption stage from 14.day with regression of the consolidations and their transformation in large GGOs. These residual changes may persist for more than four weeks.
The finding of IS predicts a clinical benefit of recruitment manoeuvre and adequate PEEP (in the sense of better O2 blood content, not in the sense of outcome). The character and distribution of IS and consolidations may predict the clinical benefit of prone positioning of the patient. We may see a resolution of pathological findings in this sequence: regression of the size of consolidation – white lung – B3 interstitial syndrome – B7 interstitial syndrome – A-profile (physiological finding).
Literature:
- Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? , Erika Poggiali et al., Radiology online, https://doi.org/10.1148/radiol.2020200847
- Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia, Fenf Pan, https://doi.org/10.1148/radiol.2020200847
- Physiotherapists use of Lung Ultrasound during the COVID-19 pandemic, practical guideline on supporting acute hospital colleagues
- Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic, Qian‑Yi Peng et al., Intensive Care Med, https://doi.org/10.1007/s00134-020-05996-6
- A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19), Yi Huang1 et al., https://ssrn.com/abstract=3544750
- Urgentní výpočetní tomografie při podezření na onemocnění COVID-19, verze 15.3.2020, Ferda et al., Ces Radiol 2020; 74(1): 577–583
- Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak, D. BUONSENSO et al., European Review for Medical and Pharmacological Sciences, 2020; 24: 2776-2780